The physician-scientists at Weill Cornell Medicine Division of Cardiology are engaged in a wide range of basic science research. Studies focus on cell biology, immunology, biochemistry, pharmacology, and genetics from bacteria to humans, as it relates to the pressing questions in the field of cardiology.
The Weill Cornell Medicine Division of Cardiology offers an integrated and collaborative environment where basic science research is highly valued. Weill Cornell Medicine offers a variety of services and development programs to support the success of scientists and investigators at all levels.
Current Basic Science Research at the Division of Cardiology
Dr. Bruce Lerman: Genetic Origins of Idiopathic Ventricular Tachycardia
Ventricular tachycardia (VT) is a potentially life-threatening rhythm disorder of the heart. While there are several different causes of VT, about ten percent of patients have no apparent structural cardiac abnormalities, in what is called “idiopathic” VT. In the most common form of idiopathic VT (RVOT), patients have no family history of VT.
Research conducted under the leadership of Weill Cornell Professor of Cardiology, Bruce Lerman, since the 1980s has suggested that RVOT is caused by a mutation in the gene for a protein called Gsα, and indeed such a mutation has been discovered in cardiac cells in Dr. Lerman’s laboratory. This is surprising, because in general the cells of the heart, unlike other organs where this mutation is found, do no continue to grow and divide after adulthood.
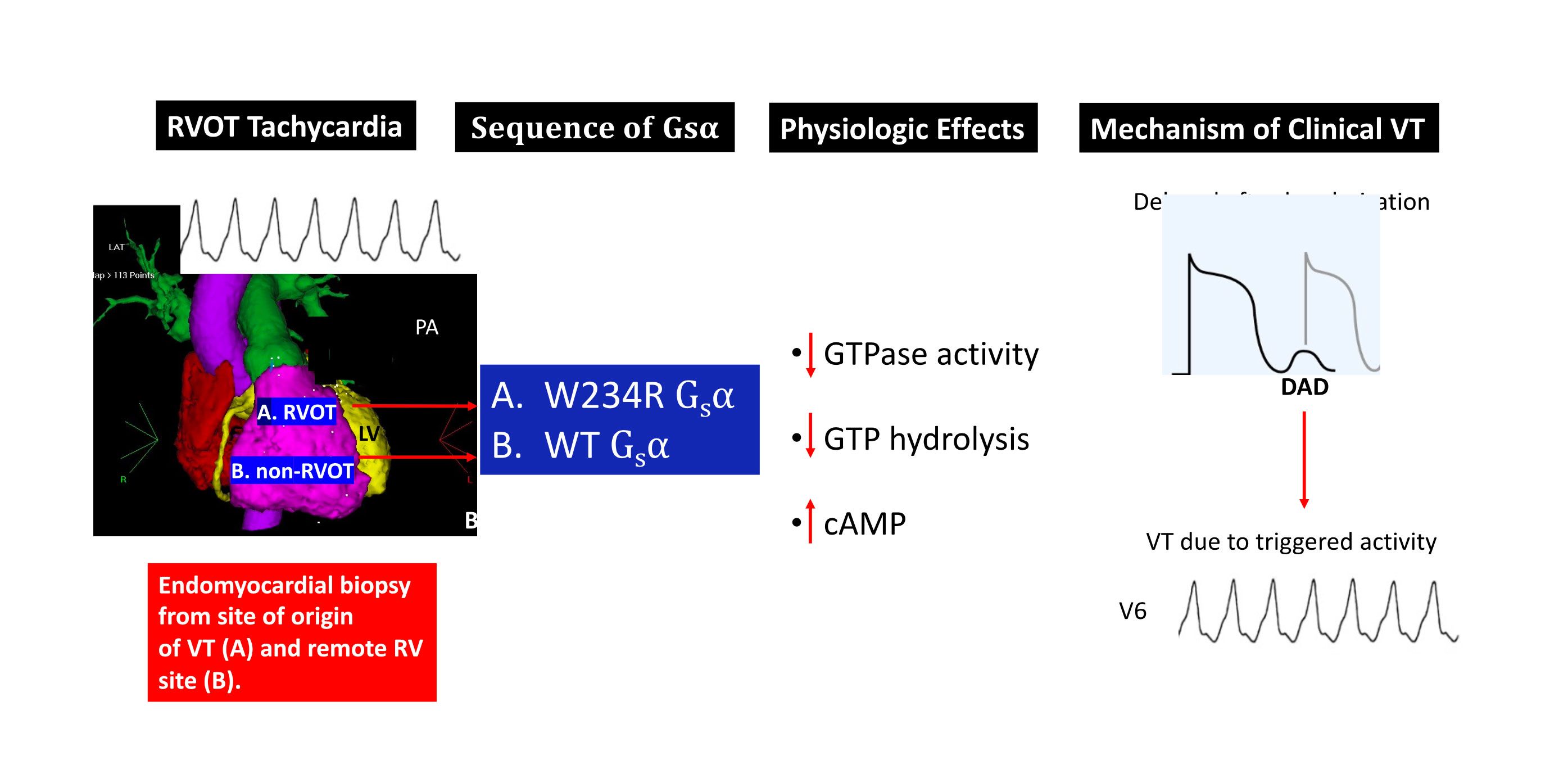
Current research, funded by the Kenny Gordon Foundation, has focused has on the cellular and molecular bases for “idiopathic” right ventricular outflow tract (RVOT) tachycardia. Although genomic mutations have been identified in ion channels and structural proteins that cause some of the less common forms of ventricular arrhythmias, the etiology often remains elusive for the most common form of idiopathic VT, that which originates from the RVOT. We have previously shown that RVOT tachycardia is caused by cAMP-mediated intracellular calcium overload that gives rise to delayed afterdepolarizations and triggered activity. Our work has also highlighted the contribution of somatic mutations to clinical cardiac disease, and demonstrates that VT, in particular, may be caused by cardiac somatic mutations in signal transductions proteins. Specifically, we have shown these arrhythmias are related to myocardial somatic mutations in the genes that encode Giα and A1AR, and GNAS, the gene that encodes the stimulatory G protein Gsα, all of which regulate intracellular cAMP. These mutations are only identified at the site of focal myocardial origin in the RVOT. Myocardial biopsies at other sites do not show the mutation, consistent with a somatic mutation rather than a germline mutation.
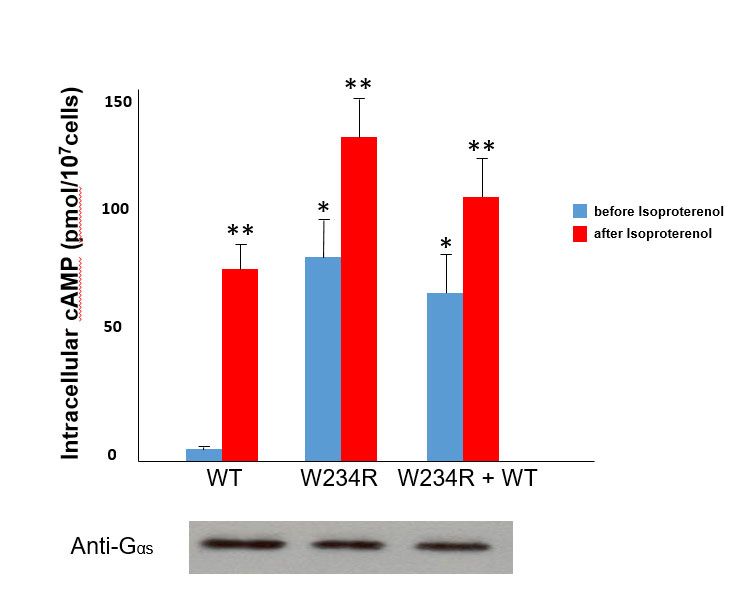
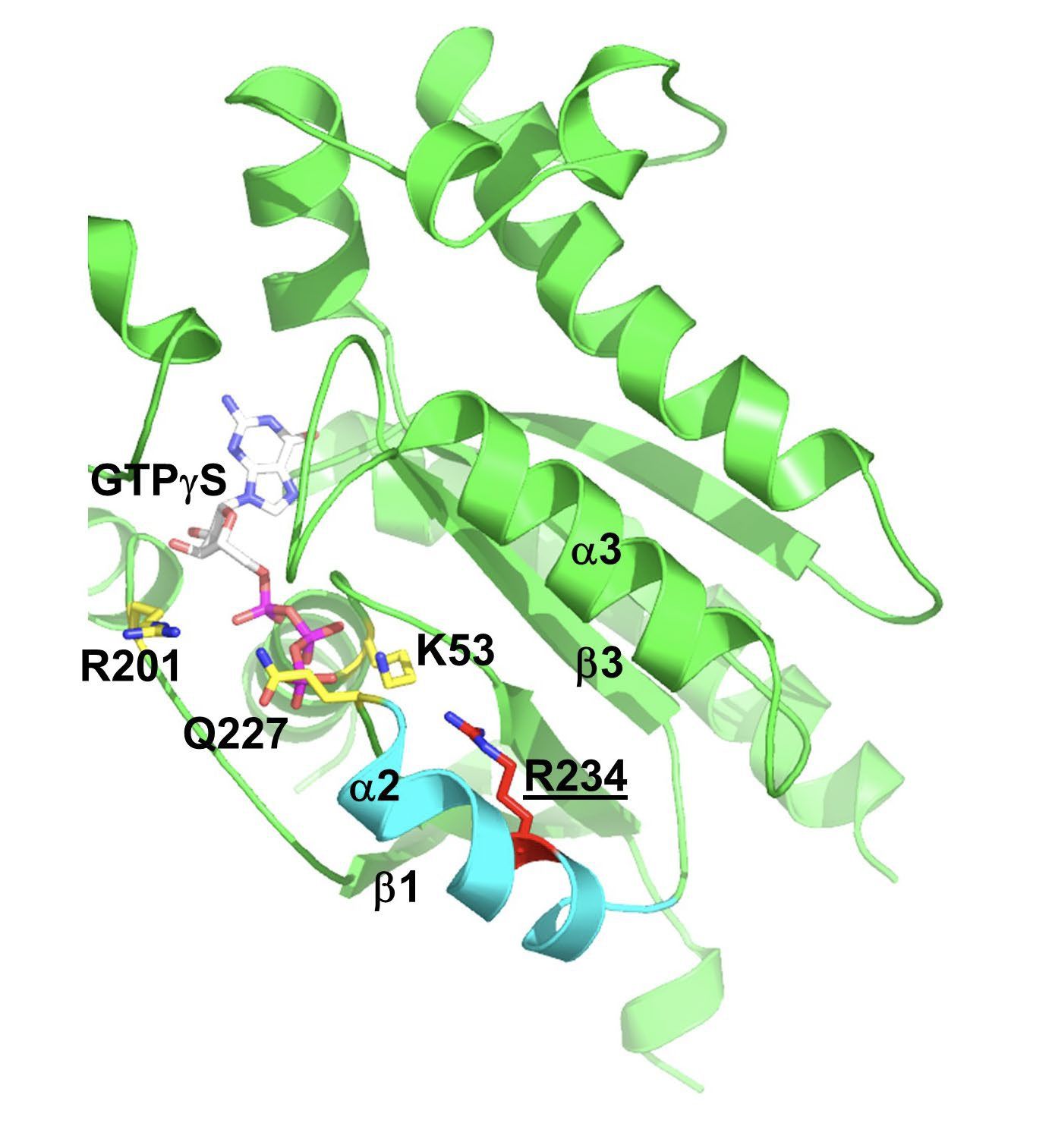
To illustrate one example, functional studies of mutant G234R Gsα (identified from a patient with RVOT tachycardia) showed a marked increase (approximately 16-fold) in basal levels of cAMP compared to cells transfected with wild-type Gsα. Moreover, the basal levels of cAMP of mutant Gsα approximated adrenergically-stimulated (via isoproterenol) levels of cAMP in wild-type Gsα transfected cells. These findings therefore suggest that the somatic mutation obtained from the site of VT origin constitutive activation of Gsα (see figure).Gsα and structural modeling predicted that the W234R mutation causes GTP binding in a conformation unsuitable for hydrolysis (figure below).
Gsα and structural modeling predicted that the W234R mutation causes GTP binding in a conformation unsuitable for hydrolysis (figure below).
Finally, whole cell patch clamp electrophysiology of mutant W234R Gsα increased L-type calcium channel current density by approximately 50%, and when this is combined with enhanced calcium current permeability from adrenergic stimulation, DADs and triggered activity were simulated in an in silico model of human ventricular myocytes. The figure below is a summary of these findings.
The laboratory is now focused on understanding the subcellular mechanisms that mediate the functional effects of the identified mutations. To that end, we are using gene editing techniques to express these mutations in induced pluripotent stem cells (iPSC) and differentiate them into cardiac cells in order to more fully understand calcium trafficking within the cell and the basis for this form of ventricular tachycardia.
Drs. Jonathan Weinsaft and Jiwon Kim: The Advanced Cardiovascular Imaging Laboratory
The Advanced Cardiovascular Imaging Laboratory develops improved methods for imaging the heart, as well as guiding therapeutic and prognostic decision making for patients with cardiovascular disease. A major focus of the lab is on use of new CMR and echocardiography techniques to elucidate links between altered myocardial tissue characteristics (i.e., changes in underlying tissue substrate) and impaired myocardial function (i.e., contraction), with the goal of developing physiologically tailored therapies to improve patient outcomes.
Translational research by the group has established links between CMR-evidenced myocardial scarring and a risk for a broad array of conditions, including heart failure, mitral valve degeneration, and stroke.
Technical research has developed new imaging algorithms, which have been shown to yield incremental utility for identification of myocardial scar, thrombus (blood clots), and cardiac dysfunction. Ongoing work by the group focuses on further improving clinical and technical including development of new high-resolution technologies to identify of micro-infarcts in critically important regions of the heart (left atrium, right ventricle) never before accessible via conventional imaging technologies.
Dr. Geoffrey Pitt: Cardiovascular Research Institute (CVRI)
This multidisciplinary center of excellence promotes accelerated translation of basic scientific advances between the bench and bedside with the goal of developing innovative therapies and diagnostic approaches to identify, prevent, and treat cardiovascular diseases. The CVRI will integrate the strong clinical cardiology programs and basic and translational research programs within the institution while building new areas of basic and translational investigation into cardiovascular diseases.
CVRI members hail from a variety of scientific backgrounds and apply diverse approaches to identify and address the molecular mechanisms of cardiovascular disease processes, such as atherosclerosis, arrhythmias, heart failure, valvular disorders, and the ravages of hypertension and obesity on the heart. We use computational, molecular, cellular, and animal models, as well as investigation of human disease with patient samples.
Cardiovascular disease remains the leading cause of death in the developed world despite the rapid advances of the past quarter century. With the aging of the world's population, we will be burdened with a growing incidence of many cardiovascular diseases prevalent in the elderly, such as atrial fibrillation, heart failure, and calcific aortic stenosis. Moreover, the rising epidemic of obesity will continue to present and increased burden of cardiovascular disease. We aim to be at the forefront to address and conquer these challenges.
Dr. James Lo: Metabolic Pathways to Disease
The Lo Lab studies the rising prevalence of obesity worldwide and its associated metabolic derangements, such as type-2 diabetes mellitus and cardiovascular disease. The primary focus is to understand the fundamental molecular mechanisms of metabolic diseases, such as diabetes, obesity, and cardiovascular disease—with the ultimate goal of developing novel treatments directed against them. We span the gamut of model systems from studying protein function in a test tube to performing in vitro cell culture to generating new mouse models and to analyzing human samples in disease states.

